Abstract
Graft versus host disease (GvHD) proceeds to be the Achilles' heel of hematopoietic stem cell transplantation, with clinicians continue facing a classic conflict: too much GvHD and the patient is at risk for transplant-related mortality and decreased quality of life; too little GvHD and the patient is at increased risk of relapse of their malignant disease. T cells and antigen presenting cells (APCs) are major components of the hematopoietic G-CSF mobilized peripheral blood cells (PBCs) graft. While GvHD is T cell mediated, the APCs are required for the initiation and maintenance of the GvHD. To reduce the risk for GvHD, grafts are sometimes depleted of their T cells, however, while preventing GvHD, the critically important attributes of graft versus leukemia (GvL) effect and engraftment are reduced significantly. Novel strategies that aim to abrogate or ameliorate GvHD, while preserving engraftment and GvL are of great need.
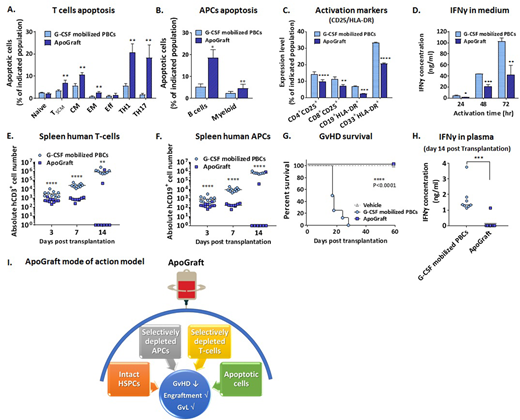
A short incubation (2hr) of G-CSF mobilized PBCs with multimeric Fas ligand (i.e. ApoGraft) selectively induces apoptosis in T cell subsets and APCs (Panels A and B), but not in CD34+ progenitor cells (data not shown). FasL treatment preferentially induces apoptosis in mature T cell subsets which express high levels of Fas (CD95), such as T stem cell memory (TSCM), T central memory (TCM), and T effector memory (TEM) cells, as well as the pro-inflammatory T cell subtypes TH1 and TH17 cells, while no apoptotic signal is detected in the non-expressing CD95 naïve T cells (Panel A). The expression of T cells and APCs activation markers; CD25 and HLA-DR, respectively, is significantly reduced following apoptotic challenge in vitro (Panel C), as well as in transplanted mice (data not shown). Furthermore, upon an activation stimulus with anti CD3/CD28 beads in vitro, ApoGraft derived T cells secrete lower levels of IFN-γ, than G-CSF mobilized PBCs derived T cells (Panel D).
To gain deeper understanding of the kinetics of GvHD development in vivo, NSG mice were transplanted with ApoGraft or G-CSF mobilized PBCs. Homing, expansion and differentiation of human leukocytes subtypes within the mice bone marrow, spleen and blood, were monitored 3, 7 and 14 days post transplantation. Decreased levels of T and B cells infiltration and expansion were detected in the spleen (Panels E and F), suggesting reduced formation of allo-reactive T cell clones. Reduced proliferation of these cells was associated with lower levels of IFN-γ secreted to the plasma (Panel H) and was in correlation with reduced GvHD and prolonged survival of the ApoGraft transplanted mice (Panel G). Importantly, we have previously demonstrated both in-vitro and in-vivo that ApoGraft has similar GvL and stem cell engraftment capabilities, compared to control G-CSF mobilized PBCs (data not shown). In conclusion, in contrast to conventional T- cell depletion methods, ApoGraft, an ex-vivo FasL-treated graft, affects both the T-cells and APCs, leading to reduced GvHD, while maintaining GvL and engraftment potential (Panel I). ApoGraft is currently being evaluated in a Phase I/II clinical trial (NCT02828878) in subjects with hematologic malignancies undergoing matched related allo-HSCT.
Levy-Barazany:Cellect Biotherapeutics Ltd: Employment. Pinkas:Cellect Biotherapeutics Ltd: Employment. Rodionov:Cellect Biotherapeutics Ltd: Employment. Marelly:Cellect Biotherapeutics Ltd: Employment. Tzadok:Cellect Biotherapeutics Ltd: Employment. Bakimer:Cellect Biotherapeutics Ltd: Employment. Yarkoni:Cellect Biotherapeutics Ltd: Employment. Peled:Cellect Biotherapeutics Ltd: Consultancy. Zuckerman:Cellect Biotherapeutics Ltd: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal